Background
Acute megakaryoblastic leukemia (AMKL) is a rare and heterogeneous subtype of acute myeloid leukemia (AML), which accounts for approximately 10% of childhood AML and about 1% of adult AML. Patients with non-Down's syndrome AMKL (non-DS-AMKL) are often associated with a poor prognosis with a 3-year overall survival (OS) rate of less than 40%. Currently, allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potential curative therapy for AMKL. However, the long-term survival post-transplant needs to be improved. Here we conducted a single-center study to evaluate the long-term efficacy and safety of allo-HSCT in 37 non-DS-AMKL patients using an innovative conditioning regimen of Busulfan/Cyclophosphamide (Bu/Cy) and Melphalan (MEL) or Thiotepa (TT).
Methods
From August 2015 to July 2023, a total of 37 non-DS-AMKL patients (M/F 21:16) were treated consecutively in our hospital (Hebei Yanda Lu Daopei Hospital). The median age was 2 years (range 1-9 years). Chromosomal abnormalities were present in 24 cases, with fusion genes as follows CBFA2T3/GLIS2 (5 cases) 13.5%, NUP98-KDM5A (2 cases) 5.4%, MLL-AF10 (3 cases) 8.1%, MLL-AF9 (1 case) 2.7%. Gene mutations: WT1 (10 cases) 27%, JAK2 (7 cases) 18.9%, EVI1 (6 cases) 16.2%, TET2 (4 cases) 10.8%, ASXL1 (4 cases) 10.8%, PTPN11 (4 cases) 10.8%, NRAS (3 cases) 8.1%, GATA1 (2 cases) 5.4%. Before transplantation, in bone marrow (BM), 29 patients were in complete remission (CR), 3 were in partial remission (PR) and the rest 5 were in non-remission (NR). Thirty-three patients received the haploidentical transplantation (donors from parents n=31, siblings n=2) including 1 with the 3 rd transplant, and 4 received matched-unrelated donor transplantation. The stem cell sources were from BM and peripheral blood stem cells (PBSCs) among the 33 related donors, and PBSCs for the 4 unrelated donors. Patients received a median cell number of total mononuclear cells 20.15×10 8/kg (10.7-32.85), CD34+ cells 12×10 6/kg(4.05-27.14) and CD3+ cells 4.66×10 8/kg(1.81-19.38).
The conditioning regimens administered were modified Bu/Cy (fludarabine 30 mg/m 2/d×5d; cytarabine 2g/m 2/d×5d; Bu 3.2 mg/kg/d×4d, Cy 1.8g/m 2/d×2d; etoposide 100mg/m 2/d×2d; antithymocyte globulin 1.5mg/kg/d×5d) in 2 patients, Bu/Cy+MEL (MEL 55mg/m 2/d×2d) in 31 patients, and Bu/Cy+TT (TT 2.5mg/kg/d×2d) in the rest 4 patients. Cyclosporine A (CSA)+ mycophenolate mofetil (MMF)+ short methotrexate (sMTX) were used as graft-versus-host disease (GVHD) prophylaxis.
Results
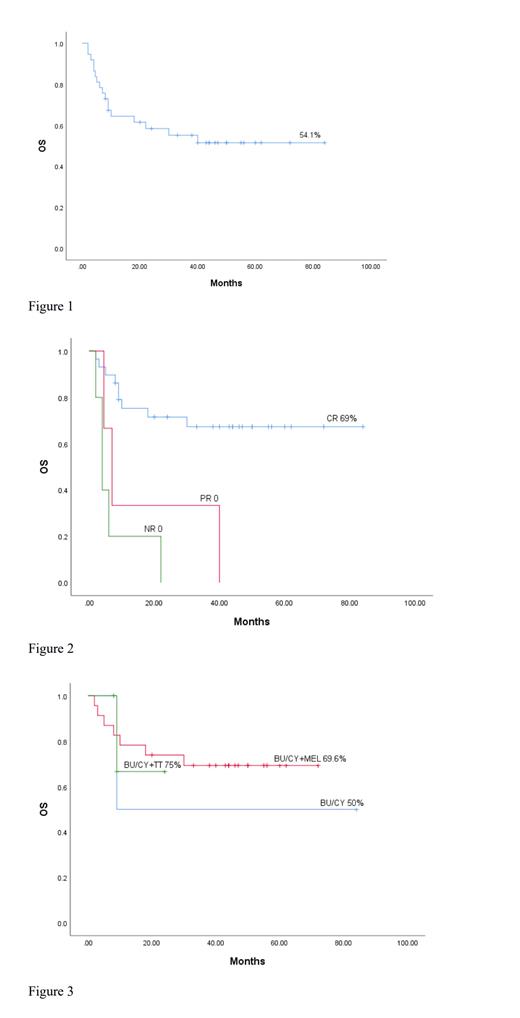
The median follow-up time was 24 months (2-84 months), and all 37 cases survived the graft, with a median leukocyte engraftment of +13 days (9-21 days), and a median platelet engraftment of +9 days (4-38 days). BM was assessed one month after transplantation, with all grafts fully donor-type. The 5-year OS was 54.1% (Figure 1). There was a significantly higher OS in the CR group (5-year OS 69%) than the PR or NR groups (P<0.001) (Figure 2). Among the CR patients, no significant difference was observed in the 3 different condition regimen groups with modified BU/CY group OS of 50%, BU/CY+MEL group OS of 69.6% and BU/CY+TT group OS of 75% (P=0.869) (Figure 3). After transplantation, 17 patients died including 11 from relapse, 4 from GVHD, 1 from gastrointestinal bleeding and 1from cerebral hemorrhage. The cumulative recurrence rate (CIR) was 29.7%, among which the CIR of the CR group was 17.2%, the PR group of 66.7% and the NR group of 80% (P<0.001). Post-transplant, the incidence of acute GVHD (aGVHD) was 35.13% (Grade II-IV), and severe aGVHD (Grade III-IV) incidence was 18.91%. And 51.35% of patients developed chronic GVHD. Other complication incidences were cystitis at 16.21%, cytomegalovirus at 5.94%, Epstein-Barr viremia at 13.5% and infections at 21.62%.
Conclusions
Our study indicates that the use of modified Bu/Cy +MEL or +TT conditioning regimens in allo-HSCT procedures presents a feasible and effective approach, demonstrating an improvement in overall survival for non-Down Syndrome AMKL patients. Crucially, it was observed that achieving CR prior to transplantation significantly contributes to better outcomes. Therefore, it is strongly recommended that allo-HSCT is performed in CR status. However, to corroborate these findings, it is essential to conduct long-term, large-scale clinical research across multiple centers.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal